Enthusiasts with a variety of interests, ranging from flashlights to electric vehicles, repurpose rechargeable lithium ion cells from laptop battery packs to power their projects. In order to assess the age and health of the cells, people use a succession of tests, ranging from quick tests of initial cell voltage, voltage ~12-24 hours after charging, and discharge tests on analyzing chargers. I looked at the initial cell voltage measurement in the context of information about pack age and history that hasn’t been available to most hobbyists.
To obtain this information, I used PackProbe. The PackProbe project allows quick access to the information contained in each pack’s “smart battery system,” using inexpensive, widely available hardware. PackProbe is part of a larger effort to improve the tools and information available to people re-using lithium ion cells.
Materials and Methods
PackProbe was used to extract data from a lot of 34 used Lenovo ThinkPad 9-cell 84Wh battery packs (IBM-42T4619) obtained from a seller on the Budget Light Forums. I don’t know more about the provenance of these packs, other than that they apparently came from a single source in a few different shipments starting sometime this summer and totaling hundreds of packs.
Observations and Commentary
General
- Most of the packs were manufactured on the 22nd and 23rd of October, 2009. Two on the 27th October, and three on the 8th of September.
- The packs all report :
- Manufactuer: SANYO06
- Chemistry: LION
- Design Capacity: 84.24 Wh
- Design Voltage: 10.800 V
- Charging Voltage: 12.600
- Charging Current: 5000 mA
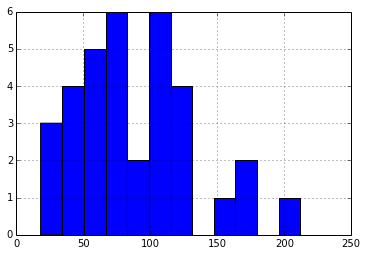
Cycle Count
The median charge/discharge cycle count is ~88, distributed as shown:
Initial Cell Voltage
We used the self-reported pack voltage from each pack to estimate initial voltage of each cell. Some of the packs had their PCBs damaged in shipment, which made it difficult to get data out of them, but by applying ~6v to the pack, I was able to read out data. The reported voltages for the damaged packs were quite low, and from my investigation, it appears that they only report the series voltage for two of the banks of cells. If I take this into consideration, and look at the average cell voltage for all the packs, I see the following distribution:
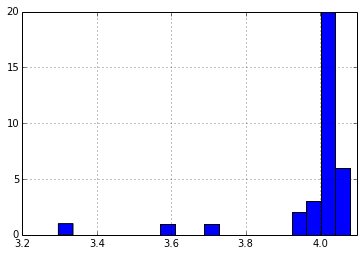
I suspect that all these packs were last charged at about the same time, likely just before their previous users gave up the machines in a round of upgrades (or layoffs). The variation in the voltages could be due to a number of factors, including variations in cell quality, pack wear, charger accuracy, storage conditions, and date of last charge.
Cell Voltage as a Proxy for Cell Wear
Exploring the idea the variations may be the result of pack wear, I’ve tried plotting cell voltage against the number of charge/discharge cycles for each pack:
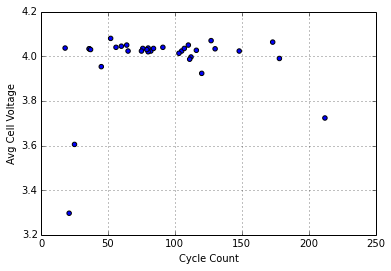
I don’t see an obvious relationship here. It seems like differences in self-discharge rate due to cell wear don’t explain the variations in voltages within the packs sampled, which all have cycle counts within the normal service life. It may be more useful for packs with large numbers of cycles (>300).
Smart Battery Capacity Measurement
SmartBattery packs maintain an estimate of pack capacity based on run-time data in order to account for aging of the pack. I wondered if initial cell voltage had a relationship to relationship to the pack management circuitry’s estimate of the packs remaining full-charge capacity:
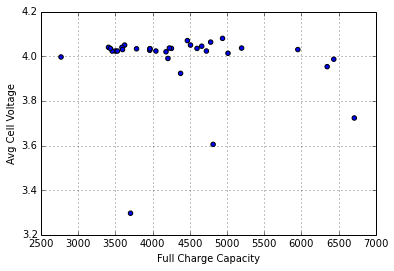
Again, I see no obvious relationship, perhaps not surprising, giving the broad distribution in the reported Full Charge Capacity (in Wh*10):
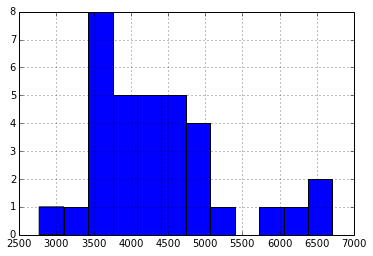
Further, the reported full charge capacity doesn’t seem to have a strong relationship to cycle count:
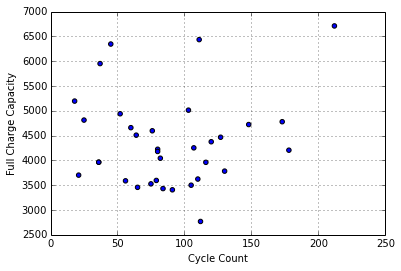
It is hard to know what to make of this, other than that there isn’t a clear relationship, which isn’t surprising, given that these packs haven’t been used for some time, and likely require a full charge/discharge/charge cycle before the full charge capacity estimates are accurate.
Discharge Test
I’ve only torn down one of these packs so far and tested the cells. Based on the tests, the full pack would have a capacity of 65 Wh, or 77% of the original capacity (at a relatively high 1C discharge rate). That’s not bad for less than $2.50, but its a pretty severe drop-off over the 103 cycles reported by the pack. It could be worse though, the reported full charge capacity was much lower, just 50Wh, or 60% of the original capacity.
Conclusions
This feels a little too much like writing up a lab report for a not-too-successful organic chemistry lab experiment back in college, lots of trying to explain inconclusive observations. Even so, I think this work has important implications.
Foremost, the results are a strong indication that initial cell voltage from used packs is not a good indicator of cell wear for used packs, at least with packs of the age and cycle count distribution of our sample.
Second, it should be clear that PackProbe provides useful information for assessing the value of a pack without necessitating tearing the pack apart.
Future Work
I intend to do more work to improve PackProbe and use it to better characterize used and aged lithium ion batteries. I hope others will join in the effort. Opportunities for community contribution include:
Join the Power Cartel forum to contribute and stay abreast of PackProbe development.


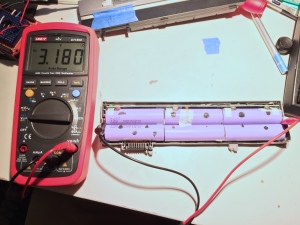







 I picked up a
I picked up a